The tetraneutron is a hypothetical stable cluster of four neutrons. However, such clusters are not supported by current models of nuclear forces, so recent news of its existence is a big deal. It means that something is not quite right about the current models.
I'm no expert in nuclear physics, and my particle theory doesn't have much to say on the matter, so I'm not going to weigh in heavily on this particular particle. However, there are nevertheless some interesting aspects related to this latest discovery that I feel competent enough to comment on.
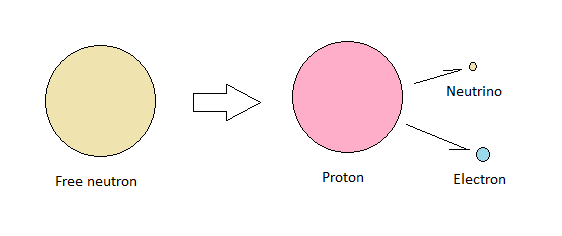
The tetraneutron is extremely unstable, with a lifetime of just 3x10-22 seconds. This compares to a single free neutron which has a typical lifespan of about 15 minutes, so putting neutrons together in clusters is not adding stability. But why is that so? How is it that atomic nuclei with overall positive charges don't blow apart, but neutral structures like the free neutron and the tetraneutron do?
The conventional answer is that there are special short range forces inside the nucleus that prevent atomic nuclei from falling apart, and that these same forces prevent neutrons from bundling into neutral structures. But this explanation is now challenged due to the short lived, but nevertheless real, tetraneutrons.
An alternative explanation presented in my work is that the electric force does not exist where there's no aether between the particles involved. Atomic nuclei are extremely dense, and can therefore be held together with electrons as glue. There's also no need for protons and electrons to be of equal number to perform this function. Each electron has two negative particle quanta, so a good mix for relatively small atoms is two protons for every electron. Larger nuclei will require more electrons to keep things together.
Translated into the language of conventional physics, we get small nuclei with roughly equal numbers of protons and neutrons, and larger nuclei with somewhat more neutrons than protons. However, my preferred model of the atomic nucleus doesn't treat neutrons as fundamental particles. Neutrons are modelled as protons with an electron attached to them.
Atomic nuclei are bundles of protons and electrons held together by the natural affinity that exists between them due to texture. Instead of short ranged forces, we have a mechanism similar to Velcro.
With little electric repulsion between particles that make up atomic nuclei, the relatively weak Velcro-like affinity that exist between negative and positive particles is sufficient to keep things together. However, the larger a nucleus becomes, the more of a role plays the electric force. This is especially so between the electrons which are spread out relative to protons.
If there's too many electrons in a nucleus, an electron may be ejected by the repelling force of other electrons in the nucleus, and we get what's known as beta decay. This loss of an electron may cause the nucleus to fall apart for lack of "glue", and we have radioactive decay in the form of fission.
Seen in this light, tetraneutron decay is a form of beta decay. The structure blows apart due to strong repelling forces between electrons.
On a related note, we can only wonder at the notion of neutron stars, which are supposedly nothing but giant balls of neutrons held together by gravity. If a single neutron decays within 15 minutes, and a structure of four neutrons collapse within 3x10-22 seconds, how is it that neutron stars remain stable for millions of years?
 |
| Free neutron decay |
No comments:
Post a Comment