Energy is size at the subatomic. Specifically, it's the surface area of subatomic particles.
When a particle is stretched or otherwise deformed, its surface increases. This translates into an increase in energy. It also translates into an increase in inertia, because inertia is related to how much time it takes to traverse a particle's surface. Hence, there's a direct relationship between energy and inertial mass.
This explains why the inertial mass of a proton is about 3 times the inertial mass of its constituent parts. Every electron and positron that makes up a proton is stretched out in such a way that their surface areas become 3 times what they are in their un-stretched state. The inertial mass of a proton is therefore about 1800 times that of an electron even though it's constructed from about 600 electrons and positrons.
We also have an explanation for why the difference in inertial mass between a neutron and a proton is more than twice that of an electron, even though a neutron is made up of exactly one proton and one electron.
The electron is stretched out across the surface of its associated proton to such an extent that it attains more than double the surface area of its free state. Neutrinos trapped inside this configuration keeps the electron from laying flat across the proton. It protrudes like a small hill.
The inertial mass of a neutron is in this way greater than that of a free electron and a free proton. The binding energy locked up in the stretching of the electron accounts for the additional inertial mass.
When a sufficiently high energy particle disturbs this arrangement, the electron is released, and it escapes from the proton at high speed together with an anti-neutrino. The binding energy is thus converted into kinetic energy.
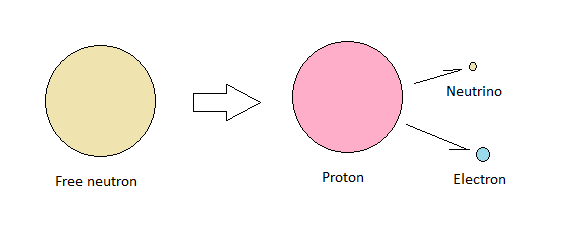 |
| Free neutron decay |
This mechanism also applies to other unstable nuclear structures, such as uranium and plutonium. Kinetic energy is released when binding energies are broken.
No comments:
Post a Comment